Chrome plating is one of the most recognizable surface treatments in the industrial world; its bright, mirror-like finish is instantly associated with strength, precision, and durability. Behind that shine, however, lies a sophisticated electrochemical process with both practical advantages and emerging challenges in modern manufacturing.
What is Chrome Metal?

Chrome metal, often referred to as chromium metal, is a metallic element with the chemical symbol Cr and atomic number 24. It is widely known for its remarkable hardness, resistance to corrosion, and high melting point.
Chrome metal is typically derived from chromite ore and is a key component in the production of alloys, most notably stainless steel. In its pure form, chromium is a shiny, silvery-gray metal that is highly durable, making it an ideal material for various industrial applications.
Here’s a detailed table summarizing the key properties of Chrome Metal:
| Property | Value/Description |
| Density | 7.192 g/cm³ (at 20°C) |
| Melting Point | 1,907°C (3,465°F) |
| Boiling Point | 2,672°C (4,842°F) |
| Hardness | 8.5 on the Mohs scale (very hard) |
| Thermal Conductivity | 94 W/m·K |
| Corrosion Resistance | Excellent (forms protective oxide layer when exposed to air) |
| Color | Silvery-gray (shiny, metallic luster) |
| Form | Solid metal; used as an alloy or for plating |
Properties of Chrome Metal:
- Hardness & Durability: Chrome is one of the hardest metals available, contributing to its widespread use in applications requiring wear resistance, such as in machinery parts, automotive components, and tools.
- Corrosion Resistance: The metal naturally forms a protective oxide layer when exposed to air, giving it excellent resistance to corrosion and oxidation. This makes it indispensable for industries like aerospace, marine, and construction.
- High Melting Point: Chrome metal has a melting point of about 1,907°C (3,465°F), which is significantly higher than many other metals. This makes it suitable for high-temperature applications, such as in furnaces or heat exchangers.
- Aesthetic Appeal: Chrome is also used for its aesthetic qualities. Its shiny, mirror-like surface is highly valued in decorative chrome plating, commonly applied to automobile parts, furniture, and appliances to enhance both appearance and durability.
Uses of Chrome Metal:
- Alloying Agent: The metal is widely used in the production of stainless steel, which contains chromium to improve its resistance to corrosion and rust.
- Tool Manufacturing: Due to its hardness, chromium is also used to create cutting tools, drill bits, and industrial machinery.
- Plating: Chrome plating is a popular surface treatment for metal and plastic parts, improving wear resistance, surface hardness, and aesthetic qualities.
In essence, chrome metal plays a pivotal role across various industries due to its exceptional mechanical properties, versatility, and resistance to harsh environments. It is essential in both functional and decorative applications.
What Is Chrome Plating?
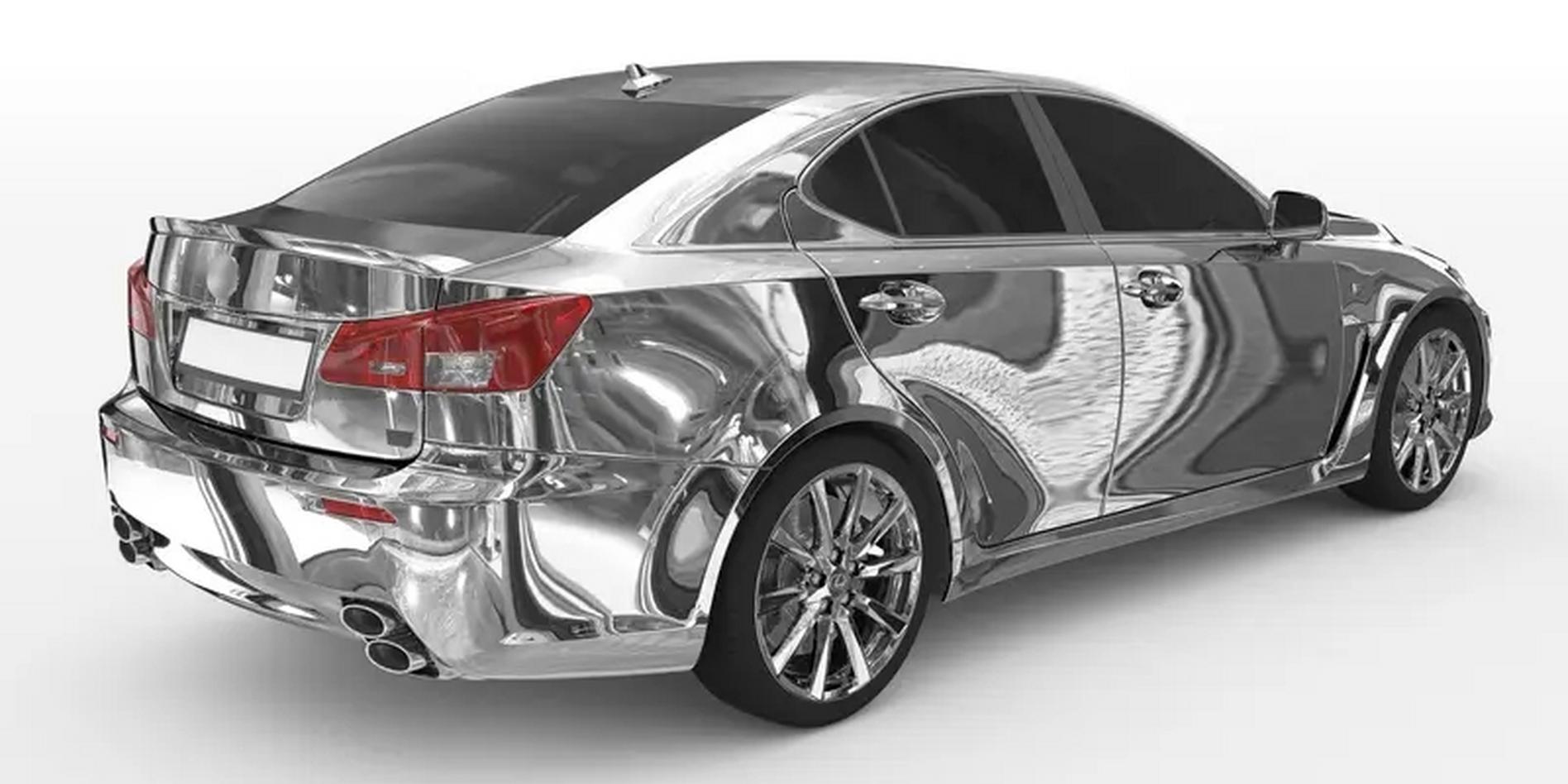
Chrome plating, also known as chromium plating, is a metal finishing process that involves applying a thin layer of chromium to the surface of a component through electroplating. This chromium layer serves both aesthetic and functional purposes, offering enhanced hardness, wear resistance, corrosion protection, and a high-gloss appearance.
Chrome plating is compatible with various base materials, including steel, stainless steel, aluminum, brass, and even certain plastics. The choice of substrate and plating thickness depends on the end-use application and required performance characteristics.
Though valued for its durability and appearance, chrome plating is increasingly examined through a broader lens, one that includes worker safety, environmental impact, and regulatory compliance.
Chroming Process Overview
Chrome plating is an electrochemical process that deposits a thin layer of chromium onto the surface of a component. The process is widely used to enhance wear resistance, reduce friction, and improve appearance.
Surface Preparation
Before any plating begins, the component must be meticulously cleaned. This step is crucial; any grease, dirt, oxide, or surface imperfection can compromise the bond between the base material and the chromium layer.
The cleaning process typically starts with alkaline degreasing to remove oil and machining residue. This is often followed by acid pickling, which strips away surface oxides and lightly etches the metal to improve adhesion. In some cases, abrasive blasting or ultrasonic cleaning is used to achieve a more uniform and reactive surface.
This phase ensures that the substrate is chemically active and physically smooth enough for the chromium to adhere evenly. Poor preparation can lead to defects such as blistering, flaking, or uneven thickness in the final product.
Electroplating Process
Once the surface is fully prepared, the component is submerged in a chromic acid-based electrolyte solution, a bath containing either hexavalent chromium (Cr⁶⁺) or the safer, increasingly adopted trivalent chromium (Cr³⁺).
An electric current is then applied. The part being plated serves as the cathode, while a conductive material, often made from lead or an inert alloy, serves as the anode. As electricity flows through the bath, chromium ions in the solution are reduced and deposited onto the part’s surface.
The characteristics of the resulting chrome layer, such as its thickness, hardness, and uniformity, are controlled by several variables:
- Current density
- Plating time
- Bath temperature
- Chemical concentration and purity
For decorative chrome plating, the chromium layer is thin (usually under 0.01 mm) and applied over a base layer of nickel, primarily to enhance appearance and protect against light corrosion. For hard chrome plating, the layer is much thicker (often between 0.05 mm and 0.25 mm) and engineered for durability, abrasion resistance, and precision tolerances.
Post-Treatment
After electroplating, the part may require post-processing to meet final specifications. This often includes polishing to achieve a desired surface finish or precision grinding to bring the part back into tolerance after the added layer of chromium.
In the case of high-strength steels, an additional step, baking at a controlled temperature, is often carried out to relieve hydrogen embrittlement, a condition that can make metal brittle after electroplating.
A final inspection and quality check ensure the chrome layer is uniform, free from defects, and bonded properly to the substrate. The result is a part that not only looks polished but also performs reliably in high-wear or corrosive environments.
Types of Chrome Plating
There are several types of chrome plating, each suited to different applications based on performance requirements.
Decorative Chrome Plating
Decorative chrome is used primarily for aesthetic enhancement and light corrosion protection. It’s commonly found on consumer-facing products such as car trim, plumbing fixtures, furniture hardware, and kitchen appliances.
This type of plating typically consists of multiple layers:
- A base layer of copper or nickel that provides corrosion resistance and smoothness.
- A very thin top layer of chromium (usually less than 0.01 mm) that gives the part its bright, reflective appearance and adds modest durability.
Because the chromium layer is so thin, the visual appeal depends heavily on the quality of the underlying nickel finish. The chrome acts mainly as a protective and oxidation-resistant topcoat.
Hard Chrome Plating
Hard chrome, also called industrial chrome or engineered chrome, is applied much more thickly and is designed for heavy-duty applications. It’s used where performance, not appearance, is the priority—such as in aerospace components, hydraulic shafts, piston rods, die-casting molds, and tooling parts.
Unlike decorative chrome, hard chrome is applied directly to the base material, often without an intermediate nickel layer. It can range in thickness from 0.05 mm to over 0.25 mm, depending on the application requirements. Also, the comparison table on decorative vs hard chrome covers the main differences.
Key characteristics include:
- High surface hardness (up to 1000 HV)
- Low coefficient of friction
- Excellent resistance to abrasion, wear, and corrosion
- Ability to be ground or honed to precise tolerances after plating
Black Chrome Plating
Black chrome plating is a specialized form of chrome plating that results in a dark, metallic black finish, often used for aesthetic, functional, or industrial purposes.
This plating process involves the electroplating of a chromium layer that has been treated with specific chemicals to produce a darkened surface, differing from traditional silver-colored chrome. Black chrome plating combines the corrosion-resistant properties of standard chrome plating with an appealing, unique appearance.
Black chrome plating is widely used across various industries for both aesthetic and functional purposes. In the automotive and consumer electronics sectors, it provides a sleek, sophisticated finish for parts such as trim, wheels, and components in high-end products.
Additionally, its durability and corrosion resistance make it a valuable choice for military, aerospace, and architectural applications where both performance and appearance are crucial.

Chrome Plating Regulations
Although chrome plating remains a widely used surface treatment, it faces growing challenges due to health regulations, environmental impact, and technical limitations. These issues are reshaping its role in modern manufacturing.
Environmental Pressures
Hexavalent chromium (Cr⁶⁺), long used in traditional chrome baths, is classified as a known carcinogen and is subject to strict environmental controls worldwide. Regulations such as EU REACH, OSHA, and EPA guidelines have imposed tighter limits on emissions, waste handling, and worker exposure, significantly increasing the cost of compliance.
Many manufacturers are now shifting toward trivalent chromium (Cr³⁺) alternatives, which are less hazardous but may not fully replicate the finish or wear resistance of hexavalent systems.
Material Constraints
Beyond regulation, chrome plating has certain functional drawbacks. The coating is brittle, prone to microcracks, and may suffer from poor coverage in deep or recessed areas. It also has limited adhesion on advanced materials like titanium or composite substrates.
Moreover, hard chrome is slow to apply, often requiring hours to build sufficient thickness and additional grinding to meet dimensional tolerances, making it less suitable for high-throughput production. Check the table sheet for a quick look at the key limitations.
| Category | Key Limitation |
| Environmental Regulation | Hexavalent chromium is toxic and heavily regulated |
| Worker Safety | Requires strict protective measures and exposure monitoring |
| Application Speed | Slow plating rate, especially for thick industrial layers |
| Coverage Uniformity | Uneven deposition in complex geometries |
| Material Compatibility | Limited adhesion on modern alloys and non-metallic materials |
| Coating Brittleness | Hard chrome is prone to cracking under cyclic stress |
Chrome Plating Alternatives
In response to tightening environmental regulations and evolving industrial needs, several alternative coating technologies have gained traction. These options aim to deliver similar or superior performance in wear resistance, corrosion protection, and surface hardness, often with fewer environmental or operational drawbacks.
| Coating Technology | Key Features | Typical Applications |
| Trivalent Chrome (Cr³⁺) | Safer chemistry, good appearance, moderate durability | Automotive trim, appliances |
| Electroless Nickel (EN) | Uniform thickness, excellent corrosion resistance | Valves, fuel systems, electronics |
| Thermal Spray Coatings | Very high wear resistance, thick layers possible | Aerospace, turbine parts, hydraulic rods |
| PVD Coating | Thin but extremely hard surface, low friction | Cutting tools, medical devices, decorative use |
| Nitriding / Nitride Coats | Surface hardening without buildup, high fatigue strength | Gears, shafts, engine components |
| Ceramic Coatings | High-temperature resistance, electrical insulation | Aerospace, electronics, extreme environments |
Chrome Plating in CNC Machining
In CNC machining, chrome plating is commonly used as a post-processing finish to enhance the durability, corrosion resistance, and wear life of precision components. It is especially valuable for parts that experience heavy friction or mechanical stress, such as hydraulic shafts, gears, and extrusion dies.
Since chrome plating deposits a thin metallic layer over the machined surface, it serves as both a protective barrier and a means to achieve specific performance characteristics, such as reduced surface friction or increased hardness.
For optimal results, engineers must factor in the plating thickness, typically ranging from 0.0002″ to 0.0005″ (5 to 13 µm), during the CNC design and tolerance planning phase. After plating, components may require precision grinding or polishing to meet exact dimensional and surface finish requirements. This is especially critical for parts with tight tolerances or sliding interfaces.
If you’re sourcing CNC machining with finishing treatments, Xmake offers professional CNC services with online quoting, helping you streamline production from prototyping to end-use parts.
Conclusion
Chrome plating remains a trusted finishing solution for enhancing the durability, wear resistance, and appearance of precision-engineered components.
As industries adapt to modern standards, their application, particularly in CNC machining, continues to evolve alongside safer and more advanced alternatives.
FAQs about Chrome Plating
Q1: Why is chrome plating banned?
Chrome plating itself is not banned, but the use of hexavalent chromium (Cr⁶⁺), a common chemical in traditional chrome plating, is heavily restricted or phased out in many countries due to its classification as a carcinogenic and environmentally hazardous substance. Regulations aim to limit exposure and emissions to protect workers and the environment.
Q2: What are the benefits of chrome plating?
Chrome plating offers several key advantages, including exceptional wear resistance, high surface hardness, low friction, and enhanced corrosion protection. It also improves the aesthetic finish of components, making it suitable for both functional and decorative applications across industries.
Q3: Is chrome plating banned in Europe?
Chrome plating is not entirely banned in Europe; however, the use of hexavalent chromium is highly regulated under the EU REACH directive. Manufacturers must obtain special authorization to use it, which has led to increased adoption of trivalent chromium and other safer alternatives in many sectors.





